Questionable Tactics Used in Vaccine Safety Testing
Story at-a-glance
By Dr. Mercola
In December 2017, Slate magazine published an astonishing article about the human papillomavirus (HPV) vaccine Gardasil, revealing how the safety trials for this controversial vaccine actually “weren’t designed to properly assess safety.”1 Gardasil is supposed to prevent infection by certain strains of HPV virus, which in rare cases may cause cervical cancer if left untreated.
However, trial data from Merck shows that Gardasil vaccinations may actually increase your risk of cervical cancer by 44.6 percent if you have been exposed to HPV strains 16 or 18 prior to vaccination.2 The U.S. Food and Drug Administration has made this document inaccessible, but we’ve saved a copy of it. In his Slate article, investigative journalist Frederik Joelving recounts the story of Kesia Lyng, a 30-year-old Danish woman who, at the age of 19, participated in a clinical trial for Merck’s Gardasil vaccine
“Lyng’s grandmother had died of cervical cancer the year before, so when a letter arrived offering her $500 to take part in a crucial international test of Gardasil, the decision was easy,” Joelving writes. “She got her first shot of the vaccine at Hvidovre Hospital in Copenhagen on September 19, 2002. The symptoms snuck up on her shortly after her second shot on November 14.
They never abated. It wasn’t until 2016 that she received her diagnosis — chronic fatigue syndrome (CFS) … In recent years, Lyng has become suspicious that there is a connection between her disease and her Gardasil immunization. Her ailments evoke descriptions found in hundreds of news stories from women who also received the vaccine, as well as several medical case reports from around the world.”
HPV Vaccine Linked to Serious Side Effects, Including Death
Reported side effects of Gardasil vaccination include immune-based inflammatory neurodegenerative disorders, suggesting something is causing the immune system to overreact in a detrimental way, sometimes fatally.3,4 The dangers of high immunogenicity was addressed in my 2015 interview with Lucija Tomljenovic, Ph.D., a research scientist at the University of British Columbia. In it, she explains that by triggering an exaggerated inflammatory immune response, vaccine adjuvants end up affecting brain function.
In collaboration with a team led by professor Yehuda Shoenfeld, a world expert in autoimmune diseases who heads the Zabludowicz Autoimmunity Research Centre at the Sheba Hospital in Israel, Tomljenovic has demonstrated how the HPV vaccine can cause brain autoimmune disorders. It was these findings that prompted the Japanese government to remove the HPV vaccine from its list of recommended vaccines.5 The vaccine injury law firm Sadaka Associates also claims that:6
“Medical researchers have accused drug regulators and manufacturers of concealing the real dangers of the HPV vaccine. Many girls have suffered life-threatening injuries as the result of the vaccine. The HPV vaccine has also caused death … The drug regulators have also been accused of adding aluminum to the placebo in order to manipulate scientific data. Even though aluminum was used in the placebo, scientists have confirmed that the HPV vaccine has been linked to death.
There was a study done that involved 2,881 girls who receive the vaccine. Fourteen of the girls who received the vaccine died. Three of the girls who received the placebo died. There was a team of researchers at the National Institute of Cardiology that also found that there is a link between HPV vaccine and life-threatening reactions.
They looked at 28 studies that involved girls who had been given the HPV vaccine. They also looked at 16 randomized trials. They found that girls were given a placebo with aluminum in 14 of the randomized trials.
If aluminum is placed in a placebo, then a person is more likely to have an adverse reaction. Spanish researchers found that girls who receive the HPV vaccine are 10 times more likely to react to it. Canadian scientists found that 10 percent of the girls who were vaccinated had to be hospitalized due to a reaction. These girls had to be hospitalized within 42 days of receiving the vaccination.”
Overstated and Unproven Effectiveness
A 2012 systematic review7 of pre- and post-licensure trials of the HPV vaccine also concluded that the vaccine’s effectiveness is both overstated and unproven. According to the authors, the review revealed:
“… evidence of selective reporting of results from clinical trials (i.e., exclusion of vaccine efficacy figures related to study subgroups in which efficacy might be lower or even negative from peer-reviewed publications). Given this, the widespread optimism regarding HPV vaccines long-term benefits appears to rest on a number of unproven assumptions (or such which are at odd with factual evidence) and significant misinterpretation of available data.
For example, the claim that HPV vaccination will result in approximately 70 percent reduction of cervical cancers is made despite the fact that the clinical trials data have not demonstrated to date that the vaccines have actually prevented a single case of cervical cancer (let alone cervical cancer death), nor that the current overly optimistic surrogate marker-based extrapolations are justified.
Likewise, the notion that HPV vaccines have an impressive safety profile is only supported by highly flawed design of safety trials and is contrary to accumulating evidence from vaccine safety surveillance databases and case reports which continue to link HPV vaccination to serious adverse outcomes (including death and permanent disabilities).”
Gardasil Safety Trials Were Not Designed to Detect Safety Problems
It’s precisely these kinds of design flaws that are highlighted in the December 17, 2017, Slate article.8 Joelving reports that Merck has repeatedly “issued reassurances about the thorough randomized trials the vaccines were subjected to before approval.”
The public was told that the three HPV vaccines marketed in the U.S. were tested on tens of thousands of individuals around the world, without any compelling evidence of serious side effects having emerged. While that reads well on paper, the shocking truth appears to be that these trials were never designed to detect and evaluate serious side effects in the first place. According to Joelving:
“An eight-month investigation by Slate found the major Gardasil trials were flawed from the outset … and that regulators allowed unreliable methods to be used to test the vaccine’s safety. Drug regulators tend to look much more seriously at potential side effects that surface during a pre-licensure study, which is what Lyng participated in, rather than after a product has already been found to be safe and been put on the market.
But regulators never learned of Lyng’s plight. In fact, her repeated complaints of debilitating symptoms were not even registered in the study as potential side effects … Lyng’s experience was not unique. Interviews with five study participants and more than 2,300 pages of documents obtained through freedom-of-information requests from hospitals and health authorities suggest inadequacies built into Merck’s major clinical tests of Gardasil.”
Joelving describes these inadequacies in great detail, showing how Merck made the vaccine appear far safer than it actually is by using “a convoluted method that made objective evaluation and reporting of potential side effects impossible during all but a few weeks of its yearslong trials.” Serious adverse events were only recorded during a two-week period post-vaccination.
Moreover, during this narrow window of time, trial investigators “used their personal judgment to decide whether or not to report any medical problem as an adverse event.”
Side Effects Simply Marked Down as Medical History
Importantly, and shockingly, most of the health problems that arose after vaccination were simply marked down as “medical history” rather than potential side effects — a tactic that basically ensured that most side effects would be overlooked. No record was made of symptom severity, duration or outcome.
Even with this gross reporting flaw, at least one Gardasil trial of the new nine-valent vaccine reported nearly 10 percent of subjects experienced “severe systemic adverse events” affecting multiple system organ classes, and over 3 percent suffered “severe vaccine-related adverse events.”9 The 2012 systematic review10 of Gardasil pre- and post-licensure trials mentioned earlier isn’t the only report out there that has offered up severe criticism of Merck’s trial tactics. Joelving writes:
“In an internal 2014 EMA report11 about Gardasil 9 obtained through a freedom-of-information request, senior experts called the company’s approach ‘unconventional and suboptimal’ and said it left some ‘uncertainty’ about the safety results. EMA trial inspectors made similar observations in another report, noting that Merck’s procedure was ‘not an optimal method of collecting safety data, especially not systemic side effects that could appear long after the vaccinations were given.'”
Study Subjects Betrayed
In other words, when Merck says Gardasil has been extensively studied for safety, it’s referring to studies set up in such a way that data on potential side effects were actually excluded. If side effects are not included in the data collection, how can you rightfully claim that no significant problems exist? Sadly, shoddy and incomplete documentation of adverse events, and follow-up periods that are too short to detect problems, can have tragic ramifications, and this is what appears to have happened with the release of Gardasil.
Joelving’s investigation reveals at least five other Danish women went on to develop debilitating health problems during the Gardasil trial. One developed severe fatigue, persistent flu-like symptoms, and had to be admitted to the hospital for a serious infection shortly after one of her vaccinations. All of her symptoms were marked down as “medical history” and were not processed as adverse events.
A year after her vaccination, she developed such debilitating pain she had to use a wheelchair. To this day, she still sometimes has to use crutches, and has been given a tentative diagnosis of psoriatic arthritis. Another young woman also developed severe fatigue and headaches. She told Joelving she reported it to study personnel, yet there’s no mention of these problems anywhere in her file. Joelving writes:
“‘If I were a research subject, I would feel betrayed,’ Trudo Lemmens, a bioethicist and professor of health law and policy at the University of Toronto, told me. ‘If the purpose of a clinical trial is to establish the safety and efficacy of a new product, whether it’s a vaccine or something else, I would expect that they gathered all relevant data, including whether it had side effects or not.'”
Imprecision Medicine
Vaccines are often riskier than oral drugs, since they’re injected into your body and contain a number of toxic adjuvants. When there’s risk, you’d expect the benefit to be worth it, but research shows many drugs provide shockingly little benefit for a majority of people, and one wonders whether the same does not hold true for vaccines as well.
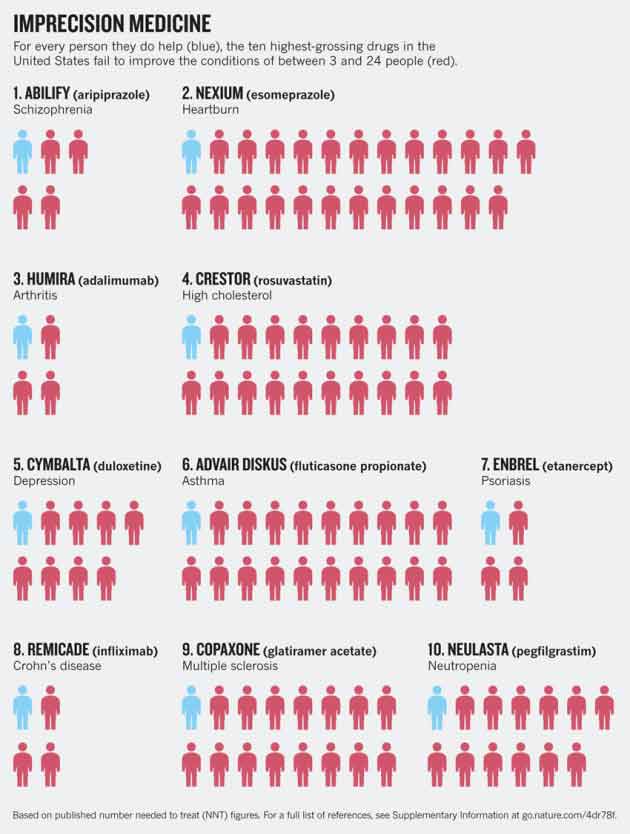
The following graphic is from a Nature article published April 29, 2015.12 It shows the effectiveness of the top 10 highest-grossing drugs in the U.S. Nexium, for example, commonly prescribed for heartburn, is beneficial for just 1 out of every 25 patients.
Advair, prescribed for asthma, helps 1 in 20; Cymbalta eases symptoms of depression in 1 out of 9 patients; Crestor, prescribed for high cholesterol, benefits 1 in 20. While not included in the graphic below, the article13 also cites research14 suggesting statins may benefit as few as 1 in 50.
Truly, when you’re talking about a benefit rate of 5 percent or less, can you really say that the drug in question is an effective one? Yet they’re certainly marketed as such. Meanwhile, all of these drugs have side effects, which means all those who gain no benefit from the drug are risking their health for no good reason whatsoever.
As noted in Nature, a wide variety of factors play into how you might respond to any given medication, including your gender, age, ethnicity and genetics giving rise to differences in absorption, metabolism, excretion and more.
“The drug vemurafenib, for instance, was approved in the United States to treat late-stage melanoma in people whose tumors carry the BRAF(V600E) mutation. But some tumor cells develop other anomalies that make them resistant to the drug. Thus clinicians considering whether to prescribe vemurafenib may need to take into account a whole slew of biomarkers,” the article states.
Pregnant Women To Be Included in Experimental Drug Trials
Historically, pregnant women have been discouraged from taking drugs and vaccines during pregnancy, as there’s very little data on their safety for the growing fetus. Pregnant women have thus far not been included in clinical drug and vaccine trials. The reason for this should be obvious.
A pregnant woman is not only putting her own health on the line, but also that of her unborn child. Now, that’s all about to change. In April 2018, the U.S. Food and Drug Administration issued draft guidance15 for industry on when and how they may include pregnant women in clinical trials for drugs and therapies. As reported by Science News:16
“It addresses considerations such as the effect pregnancy has on the absorption of drugs, nonclinical studies that should be conducted, and appropriate data collection and safety monitoring. The key concern with pregnant women participating in clinical trials is safety of the fetus.
The terrible birth defects that resulted from the wide use of the sedative thalidomide in the 1950s and ’60s weighed heavily on the eventual decision to largely exclude pregnant women from trials that test drugs. But that tragedy didn’t happen because pregnant women were studied, [obstetrician Anne] Lyerly says — it was because they weren’t studied.
‘If you don’t study a drug in a highly-controlled research setting,’ Lyerly says, ‘it’s not like the risk that would be imposed on those individuals goes away.’ Instead, the risk gets shifted to women who need the drug or women who get pregnant while on the drug.'”
According to research17 published in 2011, 94 percent of pregnant women in the study had taken one or more over-the-counter or prescription medications during their pregnancy; 70 percent used at least one prescription drug. The average number of drugs used during pregnancy has also nearly doubled in recent decades, from 2.5 in 1976/1978 to 4.2 in 2006/2008. The researchers also concluded there was insufficient data to determine the risks to the baby for 98 percent of these drugs.
While the inclusion of pregnant women in drug trials may be justifiable, as Lyerly tries to claim above, what guarantee do we have that drug companies will design studies to actually FIND side effects, opposed to doctoring studies in such a way that side effects are simply obscured?
The fact is, there are no guarantees whatsoever, as these studies will be a) done by the same companies mass-marketing drugs that are effective for 5 percent of patients or less, and b) regulated by the same government agencies that let drug companies get away with doing safety studies that don’t actually record side effects.
Safety Is a Hindrance to Profits
Getting back to the HPV vaccine, research18 shows Merck played a distinct role in state HPV vaccination policy, promoting school-entry mandates19,20,21 “by serving as an information resource, lobbying legislators, drafting legislation, mobilizing female legislators and physician organizations, conducting consumer marketing campaigns and filling gaps in access to the vaccine.”
It also found that most stakeholders thought the company “had acted too aggressively and nontransparently” to achieve their aim. Again, Merck designed their safety studies so as not to find side effects, and then aggressively lobbied to maximize vaccine uptake. So, in essence, children and teens were sacrificed in these studies just to allow the company to say they had studied the vaccine and found it safe and effective (even though it has NEVER been proven to have prevented a single case of HPV and/or cervical cancer).
And now we’re going to allow Merck and others to include pregnant women in their studies as well? What could possibly go wrong? Again and again, we see a pattern suggesting safety is not allowed to get in the way of profits and policy. History also reveals a pattern of marketing drugs and vaccines by playing on people’s fears. Most recently, Bill Gates stated he believes a global pandemic that could kill 30 million in six months is on its way, and we’re completely unprepared for it.22,23
His comments were made during an “Epidemics Going Viral, Innovation Vs. Nature” speaker series on April 27, 2018, sponsored by Massachusetts Medical Society and The New England Journal of Medicine. According to Gates, the next pandemic killer might well be a disease we’ve never encountered before.
The Bill & Melinda Gates Foundation has a history of supporting questionable vaccination agendas with their millions, so it makes sense, I guess, that Gates would be anxious to create a need for some costly remedy by amping up the fear factor. In the past decade, there’s been a string of attempts to rile up the masses and increase demand for pandemic vaccines.
The predicted pandemics all fell flat, and no mass casualties ever occurred, yet the fearmongering strategy is not easily abandoned. In the case of the HPV vaccine, it’s promoted as an anticancer vaccine, even though no proof exists that it actually prevents cancer. As mentioned earlier, Merck’s own research revealed an increased risk of cervical cancer with the vaccine under certain circumstances.
The Dangers of HPV Are Overhyped — Understand What You’re Vaccinating Against and What the Alternatives Are
It may be worth remembering the basics when pondering the decision of whether or not to vaccinate your child against HPV:
• There are over 200 viral strains of HPV. Gardasil 9, licensed in 2015, contains the original Gardasil HPV types 16, 18, 6 and 11, plus types 31, 33, 45, 52 and 58, which are associated with cervical, vulvar, vaginal and anal cancers. Cervical cancer accounts for less than 1 percent of all cancer deaths in the U.S. and anal cancer kills approximately 300 Americans each year. So, HPV vaccine is not targeting a major public health threat, no matter which way you look at it.
• Most HPV cases are in fact harmless, and your immune system is typically able to fight and clear out the infection naturally, even without treatment. In 90 percent of cases, HPV resolves within two years or less; 70 percent clear within one year. In a small percentage of individuals, HPV can persist for years, and may cause symptoms to appear, particularly when the immune system weakens. High-risk HPV strains may also cause lesions that sometimes can evolve into cervical cancer if left untreated.
• To avoid contracting HPV, use condoms during sexual activity. Research24 has demonstrated that using condoms can reduce the risk of HPV infection by 70 percent, which is far more effective than the HPV vaccine. If you have children nearing sexual maturation, teach them about the importance of safe sex — not just for the avoidance of HPV, but also to avoid other sexually transmitted diseases, many of which are now resistant to antibiotics and exceptionally difficult to treat.
• Get regular Pap smears once sexually active, and get treatment if testing positive for HPV infection. Remember, it’s the long-term, untreated infections that can trigger cancer. According to research published in 2014, shiitake mushroom extract can speed up the elimination of HPV infection in women by boosting immune function.
Routine Pap smear testing is a far more rational, less expensive, and less dangerous strategy for cervical cancer prevention, as it can identify chronic HPV infection and may provide greater protection against development of cervical cancer than blind faith in an unproven HPV vaccine.
Disclaimer: The entire contents of this website are based upon the opinions of Dr. Mercola, unless otherwise noted. Individual articles are based upon the opinions of the respective author, who retains copyright as marked. The information on this website is not intended to replace a one-on-one relationship with a qualified health care professional and is not intended as medical advice. It is intended as a sharing of knowledge and information from the research and experience of Dr. Mercola and his community. Dr. Mercola encourages you to make your own health care decisions based upon your research and in partnership with a qualified health care professional. If you are pregnant, nursing, taking medication, or have a medical condition, consult your health care professional before using products based on this content.
